If we consider the skin to be a shield, barrier or a protective layer, it is easy to understand why it is essential to maintain good skin integrity to avoid complications.
Monitoring skin integrity is a nursing skill that is as important as any other, especially in the case of patients with vascular access devices.
Therefore, to help us to better manage vascular access, this article contains some recommendations to avoid medical adhesive related skin injuries (MARSIs).
SKIN FUNCTION
The skin is a complex and dynamic human organ. Indeed, it is our largest, most extensive, and visible organ, covering almost 2m² and accounting for almost 1/6 of our body weight. It protects us from damage, acts as a barrier, maintains our temperature and is our shield. Moreover, it is a living and changing organ that evolves and is subjected to biological, biomolecular, physiological and chemical processes.
Obviously, despite having common characteristics in all patients, the approach to skin care—clearly a nursing skill—must be done on an individual basis.
WHAT ARE MARSIs?
General definition
According to Laurie McNichol, MARSIs (medical adhesive related skin injuries) are “any alteration in skin integrity characterized by erythema and/or other skin damage including skin tears, erosion, bulla, or vesicle that persists for 30 minutes or more after removal of a medical device containing adhesive (1).” (Figure 1).

A medical adhesive is considered to be any substance that has the ability to adhere to the skin. This category includes the dressings used to fix vascular catheters to it.
MARSI in vascular access
In the specific case of MARSIs related to vascular access, the injuries may entail an increase in management cost and time. They can also lead to an increased risk of catheter infection and accidental removal. (3)(4)
All this results in a reduction in patients’ quality of life as they may experience pain, discomfort, or concern about the device and their skin lesion.
With regards to MARSIs related central venous accesses, in 2017, Hitchcock and Savine(2) defined a prevention-action algorithm for such injuries (Figure 2) based on the MARSI classification previously published by McNichol and colleagues(1) in 2013.

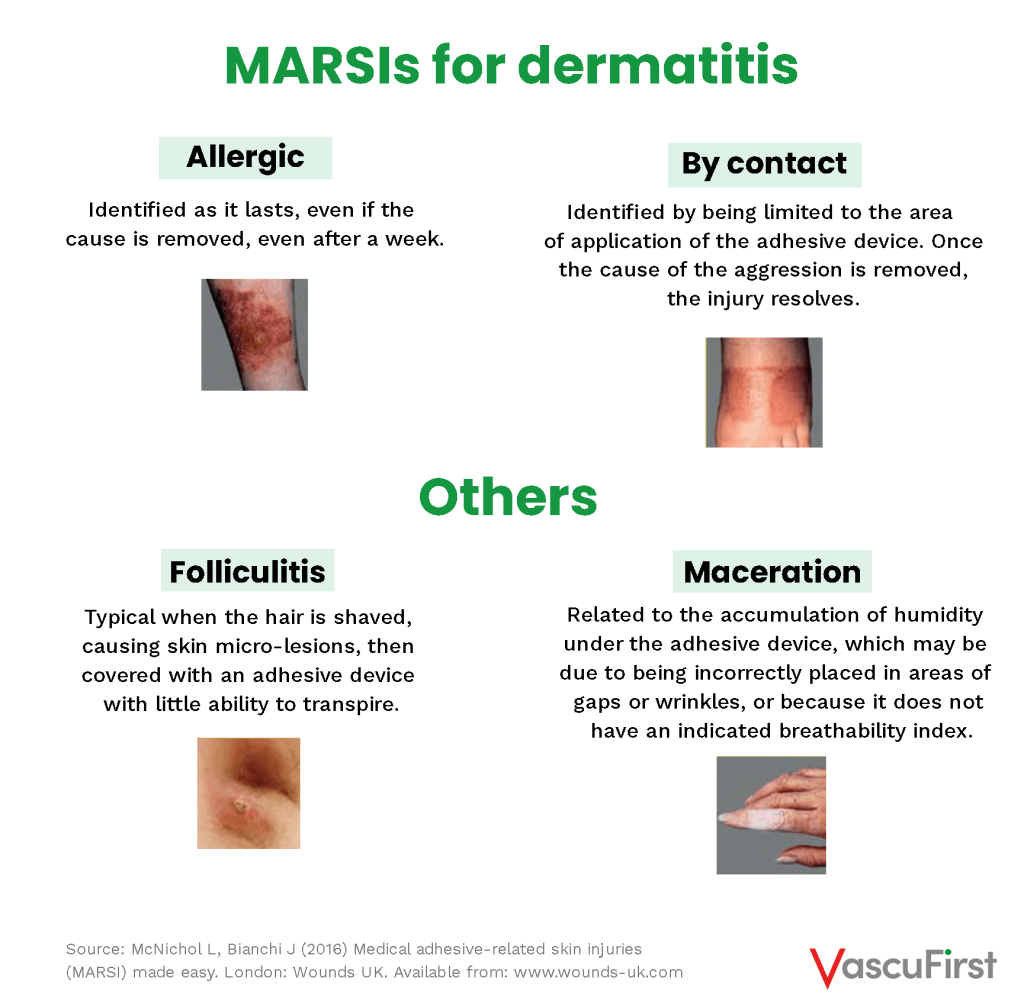
MARSI CLASSIFICATION
This simple but useful McNichol classification differentiates between (5):

FACTORS DETERMINING THE ONSET OF MARSI
We know now that these injuries, irrespective of their classification, depend on various intrinsic and extrinsic factors to the patient (3)(6).
These factors have a positive or negative influence and are sometimes difficult, if not impossible, to control or modify. They include:
- Intrinsic factors: dehydration, eczema, dermatitis, immunosuppression, age, race, allergies, infection, critical care situation, radiation, diabetes, malnutrition, vascular diseases, among others
- Extrinsic factors: chemotherapy, anticoagulant treatment, climate, exposure to extreme temperatures, etc.
Since these factors are not very susceptible to variations by healthcare personnel, we must focus, as always, on prevention (1)(7)(8).
We need to consider these factors to select the right strategy (4). They undoubtedly include the choice of a stabilisation device in terms of both the type of adhesive and the structure that contains it.
A vascular access dressing that prevents or reduces the development of MARSIs should be the least traumatic to achieve the required fixation:

In any case, we cannot forget the dressing placement-removal technique, as summarised in the following simple steps (it may be a good idea to create a bundle):

Medical adhesive devices must be selected with care based on the purpose that they must fulfil the type of structure required to minimize tissue damage and each patient’s skin type (4). There are numerous ways of fixing VADs to the skin such as engineered suture-less securement devices. Based on scientific evidence, the use of sutures was discouraged and there are now many similar devices available on the market. Their role is to prevent catheter movement and inadvertent dislodgement. Several types are available, with different flexibility, sizes, prices, and structures. However, in empirical scientific studies, their superiority over sutures has been demonstrated (9)(10)(11). The remaining challenge is to reduce the damage to the skin caused by the medical adhesive they contain.
Some recent studies have begun to shed light on the use of suture-less and subcutaneous catheter fixation devices, products that dispense with medical adhesives completely (11)(12)(13). Considering these results and the fact that skin integrity is an essential care for vascular access patients, the question then arises spontaneously: could suture-less fixation devices lacking medical adhesive be the future of vascular access fixation?
REFERENCES:
1- McNichol L, Lund C, Rosen T, Gray M. Medical adhesives and patient safety: state of the science: consensus statements for the assessment, prevention, and treatment of adhesive-related skin injuries. J Wound Ostomy Continence Nurs. 2013 Jul-Aug;40(4):365-80; quiz E1-2. doi: 10.1097/WON.0b013e3182995516. PMID: 23759927.
2- Hitchcock J, Savine L. Medical adhesive-related skin injuries associated with vascular access. Br J Nurs. 2017 Apr 27;26(8):S4-S12. doi: 10.12968/bjon.2017.26.8.S4. PMID: 28453313.
3- Ousey K, Cooper K, Fumarola S, Hitchcock J.
Findings from a multidisciplinary focus group meeting to discuss the issue of medical adhesive-related skin injury (MARSI) in the UK: The way forward. Wounds UK. 2017.13. 141-145.
4- Broadhurst D, Moureau N, Ullman AJ; World Congress of Vascular Access (WoCoVA) Skin Impairment Management Advisory Panel. Management of Central Venous Access Device-Associated Skin Impairment: An Evidence-Based Algorithm. J Wound Ostomy Continence Nurs. 2017 May/Jun;44(3):211-220. doi: 10.1097/WON.0000000000000322. PMID: 28353488; PMCID: PMC5417573.
5- McNichol L, Bianchi J. (2016) Medical adhesive-related skin injuries (MARSI) made easy. London: Wounds UK. Available from: www.wounds-uk.com
6- Zhao H, He Y, Wei Q, Ying Y. Medical Adhesive-Related Skin Injury Prevalence at the Peripherally Inserted Central Catheter Insertion Site: A Cross-sectional, Multiple-Center Study. J Wound Ostomy Continence Nurs. 2018 Jan/Feb;45(1):22-25. doi: 10.1097/WON.0000000000000394. PMID: 29300286.
7- LeBlanc K, Whiteley I, McNichol L, Salvadalena G, Gray M. Peristomal Medical Adhesive-Related Skin Injury: Results of an International Consensus Meeting. J Wound Ostomy Continence Nurs. 2019 Mar/Apr;46(2):125-136. doi: 10.1097/WON.0000000000000513. PMID: 30844869; PMCID: PMC6519893.
8- Yates S, McNichol L, Heinecke SB, Gray M. Embracing the Concept, Defining the Practice, and Changing the Outcome: Setting the Standard for Medical Adhesive-Related Skin Injury Interventions in WOC Nursing Practice. J Wound Ostomy Continence Nurs. 2017 Jan/Feb;44(1):13-17. doi: 10.1097/WON.0000000000000290. PMID: 28060000.
9- Dolcino A, Salsano A, Dato A, Disma N, Pini Prato A, Bernasconi F, Montagnini L, Avanzini S, Bevilacqua M, Montobbio G, Mattioli G, Zanaboni C. Potential role of a subcutaneously anchored securement device in preventing dislodgment of tunneled-cuffed central venous devices in pediatric patients. J Vasc Access. 2017 Nov 17;18(6):540-545. doi: 10.5301/jva.5000780. Epub 2017 Aug 2. PMID: 28777409.
10- Egan GM, Siskin GP, Weinmann R 4th, Galloway MM. A prospective postmarket study to evaluate the safety and efficacy of a new peripherally inserted central catheter stabilization system. J Infus Nurs. 2013 May-Jun;36(3):181-8. doi: 10.1097/NAN.0b013e3182893690. PMID: 23558917.
11- Pittiruti M, Scoppettuolo G, Dolcetti L, Celentano D, Emoli A, Marche B, Musarò A. Clinical experience of a subcutaneously anchored sutureless system for securing central venous catheters. Br J Nurs. 2019 Jan 24;28(2):S4-S14. doi: 10.12968/bjon.2019.28.2.S4. PMID: 30673323.
12- Zerla PA, Canelli A, Cerne L, Caravella G, Gilardini A, De Luca G, Aricisteanu AM, Venezia R. Evaluating safety, efficacy, and cost-effectiveness of PICC securement by subcutaneously anchored stabilization device. J Vasc Access. 2017 May 15;18(3):238-242. doi: 10.5301/jva.5000655. Epub 2017 Feb 15. PMID: 28218360.
13- Goossens GA, Grumiaux N, Janssens C, Jérôme M, Fieuws S, Moons P, Stas M, Maleux G. SecurAstaP trial: securement with SecurAcath versus StatLock for peripherally inserted central catheters, a randomised open trial. BMJ Open. 2018 Feb 24;8(2):e016058. doi: 10.1136/bmjopen-2017-016058. PMID: 29478011; PMCID: PMC5855473.





0 Comments
Trackbacks/Pingbacks