Introduction
The need for vascular access to administer intravenous medicines and fluids is an essential part of healthcare delivery in acutely ill patients.1 It is suggested that most patients admitted to acute hospitals will have at least one vascular access device (VAD) inserted during their hospital stay.2 The use of VADs is not without risks with catheter related blood stream infections (CRBSI) described as potentially the most dangerous complications associated with healthcare.1 In the last European point prevalence study of healthcare associated infections (HCAI), 10.8% of the HCAIs were bloodstream infections (BSI).3 Previous studies have shown that over 60% of HCAI BSI are in patients with a vascular access device.1 The risk of CRBSI infection is significantly higher in patients with a CVAD compared to peripheral intravenous cannula (PIVC).4 Beside the risk of infection and associated mortality risk, CRBSI have an economic impact with increased hospital length of stay and increased costs.3,5 The cost of CRBSIs is estimated to range between $33 000 and $75 0006 with the length of stay extended, on average, by almost 12 days for patient with a BSI5. To prevent vascular access device infections, we must first understand the chain of infection.
The chain of infection
The term ‘chain of infection’ has evolved as a way of describing the sequence of events necessary for an infection to occur. Understanding the chain of infection related to vascular access allows one or more links of the chain to be broken and therefore preventing infection to occur.
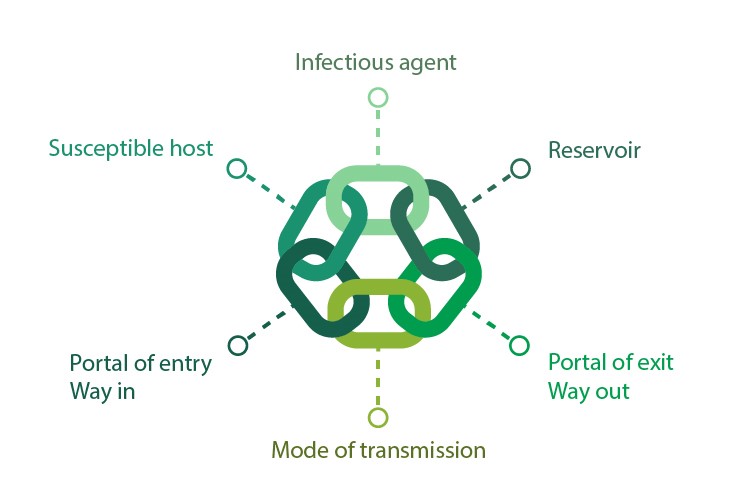 Image 1 – Chain of Infection
Image 1 – Chain of Infection
The Chain of Infection related to vascular access device infections
Infectious agents – although there are many microorganisms that can cause VAD-related infections, the commonest is coagulase negative Staphylococcus, particularly S. epidermidis but others include Staphylococcus aureus, Enterocococcus and Candida species.1 S. aureus and S. epidermidis are both skin commensals and can be present on the patient’s skin or the hands of the healthcare worker.8 Gram negative organisms that live in the gut such as Pseudomonas spp., Klebsiella spp., Enterobacter spp, Stenotrophomonas maltophilia, Burkholderia cepacia and Citrobacter freundii can also cause vascular access device infections.8,9
Reservoir – the reservoirs can include the environment, people and equipment. Damaged packaging of sterile equipment and contaminated intravenous stands and pumps is referred to as intrinsic contamination. Contamination of the catheter hub and port or contamination of the intravenous fluids is classed as extrinsic contamination.8 The Gram-negative organisms are predominately found in the human gut but can be found harbouring in hand wash basin outlets providing a significant reservoir in some clinical areas.10
Portals of exit – the microorganisms can leave the body through secretions of body fluids, respiratory droplets or skin.8 These microorganisms can then contaminate the surface and equipment which contribute to the reservoir as described above.
Mode of transmission – Contact transmission is the most common means of transmission. Direct contact transmission occurs when there is physical contact between a contaminated surface and a susceptible person, for example contamination from patient’s bedding and then accessing the vascular access device without hand decontamination.11 Indirect contact transmission occurs when microorganisms are moved from a reservoir to contaminated surfaces or objects for example Pseudomonas spp. spread via aerosols from a contaminated sink to a preparation surface where IV drugs are being prepared.10,12
Portal of entry – this is the point where the microorganism can enter the host. In intravenous therapy there are four possible routes for entry.8,13
- Extraluminal is where the microorganisms can migrate down the catheter tract where the skin’s integrity has been breached to insert the vascular access device. These microorganisms can be from the healthcare works hands, the patient’s skin or contaminated disinfectant or ultrasound gel.
- Intraluminal refers to contamination via the catheter hub usually by contaminated hands accessing the vascular access device for drug administration, connection of fluids or withdrawal of blood via the catheter hub.
- Contaminated infusate which can either occur during the manufacturing process or during manipulation by the healthcare worker.
- Haematogenous seeding from bacteria circulating in the bloodstream from another body site that attaches to the catheter surface. This could be from an infected wound or urinary tract because of urinary catherization.
 Image 2: Routes of entry
Image 2: Routes of entry
Susceptible host – any break in the skins natural defence mechanism increases a person’s susceptibility to infection. Extremities of age, underlying diseases such as diabetes and cancer and medications such as cytotoxic drugs can weaken the immune system and leave some patients even more susceptible to infection.8,9
Microbial Contamination
The microbial contamination leads to some of the bacteria excreting extracellular polysaccharides which produce a biofilm. The biofilm is a collection of microbial cells that become attached to the surface of the catheter and are encased in a self-produced matrix providing the idea environment for multiplication of the bacteria.4 One of the growing concerns is the use of gloves during vascular access procedures with observational studies highlighting missed opportunities for hand decontamination.11,14 Dunn and colleagues14 describe a quality improvement programme to educate and empower frontline staff to use gloves appropriately and improve compliance with hand hygiene. The results of this programme saw that staff were making good risk assessments on the use of gloves when preparing and administering intravenous drugs thus removing barriers to hand hygiene.14 Moreover, this programme saw a 30% reduction in glove purchasing with saving of £90,000 along and a reduction of around 18 tonnes of waste in the first year.14 Curran12 and Garvey10 both highlighted the potential risk of contamination from hand wash basins by the aerosols created during hand washing. Consideration of the location and proximity of the hand washbasin is important to prevent contamination of the surfaces and equipment when preparing medicines for intravenous administration.
Conclusion
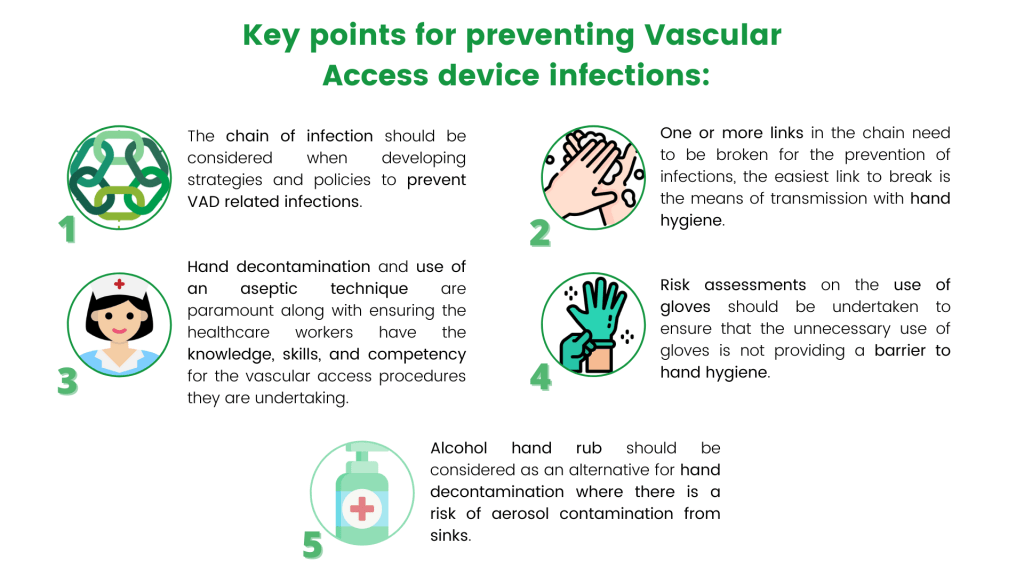
Image 3: Key Points for preventing Vascular Access device infections
References
– Loveday HP, Wilson JA, Pratt RJ, et al. (2014a). Epic3: National Evidence –Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals. Journal of Hospital Infection. S86, ppS1-S70
– Alexandrou E, Ray-Barruel G, Carr P, et al. OMG Study Group (2018) Use of Short Peripheral Intravenous Catheters: Characteristics, Management, and Outcomes Worldwide. Journal of Hospital Medicine. Online E1-E7
– Suetens C., Latour K., Kärki T., the Healthcare-Associated Infections Prevalence Study Group et al (2018) Prevalence of healthcare associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017 Euro Surveill.2018;23(46): https://doi.org/10.2807/1560-7917.ES.2018.23.46.1800516
– Gominet M, Compain F, Beloin C, et al. (2017) Central venous catheters and biofilms: where do we stand in 2017? APMIS 2017; 125: 365–375. DOI 10.1111/apm.12665
– Stewart S., Robertson C., Pan J., et al (2021) Impact of healthcare associated infection on length of stay. Journal of Hospital Infection. 114:23-31 https://doi.org/10.1016/j.jhin.2021.02.026
– Hollenbeak C. (2013) The cost of catheter-related bloodstream infections: implications for the value of prevention. Journal of Infusion Nursing. 34:309–13.
– Fakih M., Bufalino A., Sturm L., et al (2021) Coronavirus disease 2019 (COVID-19) pandemic, central-line–associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts Infection Control & Hospital Epidemiology 1–6 https://doi:10.1017/ice.2021.70
– Lavery (2010) Infection control in IV therapy: a review of the chain of infection British Journal of Nursing, (Intravenous Supplement). 19(19):6-14
– Carr P, Rippey J, Cooke M, et al. (2016) Development of a clinical prediction rule to improve peripheral intravenous cannulae first attempt success in the emergency department and reduce post insertion failure rates: the Vascular Access Decisions in the Emergency Room (VADER) study protocol. British Medical Journal Open https://.doi:10.1136/bmjopen-2015-009196
– Garvey M., Wilkinson M., Holden K. (2019) Tap out: reducing waterborne Pseudomonas aeruginosa transmission in an intensive care unit. Journal of Hospital Infection 102(1):75-81. https://doi:10.1016/j.jhin.2018.07.039
– Loveday H., Lynam S., Singleton J. et al (2014b) Clinical glove use: healthcare workers’ actions and perceptions. Journal of hospital infection. 86(2):110-6.https://doi:10.1016/j.jhin.2013.11.003
– Cunnan (2013) Intravenous drug preparation: the infection risks. British Journal of Nursing https://doi.org/10.12968/bjon.2011.20.Sup7.S4
– Zhang L., Cao S., Marsh N., et al (2016) Infection risks associated with peripheral vascular catheters. Journal of Infection Prevention. Vol. 17(5) 207–213 https://DOI:10.1177/1757177416655472
– Dunn H., Wilson N., Leonard A. (2019) A programme to cut inappropriate use of non-sterile gloves. Nursing Times. 115(9): 18-20




0 Comments